FDA recall: Teva prazosin blood-pressure capsules flagged for impurity
post on 30 Oct 2025
post on 30 Oct 2025
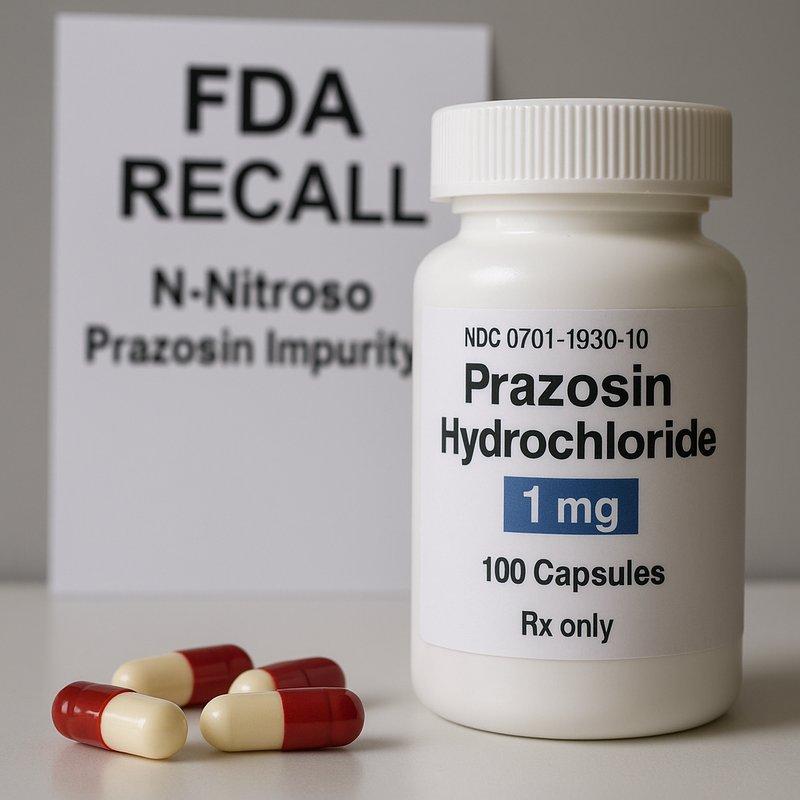
FDA recall: prazosin capsules.
30 October 2025
Teva Pharmaceuticals has voluntarily recalled more than 580,000 bottles of prazosin hydrochloride capsules after tests detected a nitrosamine contaminant, identified as N-nitroso prazosin impurity C. The US Food and Drug Administration (FDA) has classified the action as a Class II recall (risk of temporary or medically reversible harm; serious effects are unlikely).
Strengths: 1 mg, 2 mg, 5 mg
Bottle counts affected (FDA/press tallies): 181,659 (1 mg), 291,512 (2 mg), 107,673 (5 mg) — ≈580,844 bottles total.
Distributor: Teva Pharmaceuticals USA (Parsippany, NJ).
Prazosin relaxes blood vessels to lower blood pressure and is also prescribed off-label for PTSD-related nightmares.
Nitrosamines are a broad class of chemicals; long-term exposure above acceptable limits may increase cancer risk, though the FDA does not expect harm at low levels. The agency sets nitrosamine acceptable intake limits (including for NDSRIs) using its Carcinogenic Potency Categorization Approach (CPCA).
Don’t stop the medicine on your own. For Class II recalls, the FDA states that patients can generally continue their medication unless the company or their clinician instructs them otherwise. Contact your pharmacist/prescriber to arrange a verified replacement if your bottle is part of the recall.
Check identifiers. Teva’s prazosin bottles list NDCs such as 00093-4067 (1 mg), 00093-4068 (2 mg), 00093-4069 (5 mg); your pharmacy label shows the NDC and lot they dispensed. (NDC alone does not confirm a recall—lot matters.)
Find lot/“code information”. Search the FDA’s Enforcement Reports database (look under Drugs) or ask your pharmacist to check the lot against the recall.
Report problems. Suspected side effects or product-quality issues can be reported to the FDA's MedWatch program (online form or FDA Form 3500/3500B).
Recall initiated: 7–8 Oct 2025 (company/agency notices recorded that week).
FDA classification: Class II, posted 24 Oct 2025.
Impurity: N-nitroso prazosin impurity C (nitrosamine).
Since 2018, regulators have tightened surveillance for nitrosamines across multiple medicines and now publish intake limits and control strategies for manufacturers. Patients should work with their pharmacy/clinician to switch affected lots and avoid abrupt interruptions in chronic therapy.
Verify lot numbers against FDA Enforcement Reports and distributor notices; quarantine affected stock and follow reverse-logistics instructions.
Counsel patients not to self-discontinue antihypertensives; arrange like-for-like replacement where needed.
Related News: Breaking: FDA Orders Major Atorvastatin Recall 2025, 140,000 Bottles Affected
Check the lot number (and NDC) on your pharmacy label or bottle, and then ask your pharmacist to verify it against the FDA’s Enforcement Reports.
No—don’t discard or stop taking it on your own. Contact your pharmacist/clinician first; for Class II recalls, FDA advises patients can generally continue until a replacement is arranged.
Submit a report to FDA MedWatch (consumer or clinician form) and notify your prescriber.